The pH Scale
Hydronium ion concentration in molarity is more conveniently expressed on a logarithmic scale known as the `color{red}(pH)` scale.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
The `color{red}(pH)` of a solution is defined as the negative logarithm to base 10 of the activity `color{red}(a_(H^(+))` of hydrogen ion.
In dilute solutions `color{red}(< 0.01 M)`, activity of hydrogen ion `color{red}((H^+))` is equal in magnitude to molarity represented by `color{red}([H^+])`. It should be noted that activity has no units and is defined as:
`color{red}(a_(H^(+)) = [H^+]// mol L^(-1))`
From the definition of `color{red}(pH)`, the following can be written,
`color{red}(pH = -loga_(H^+) = - log { [H^+] // mol L^(-1) })`
Thus, an acidic solution of `color{red}(HCl (10^(–2) M))` will have a `color{red}(pH = 2).` Similarly, a basic solution of `color{red}(NaOH)` having `color{red}[OH^– ] =10^(–4) M)` and `color{red}([H_3O^+] = 10^(–10) M)` will have a `color{red}(pH = 10).`
At `25 °C`, pure water has a concentration of hydrogen ions, `color{red}([H^+] = 10^(–7) M)`. Hence, the `color{red}(pH)` of pure water is given as:
`color{red}(pH = - log (10^(-7) ) = 7)`
`=>` Acidic solutions possess a concentration of hydrogen ions, `color{red}([H^+] > 10^(–7) M)`, while basic solutions possess a concentration of hydrogen ions, `color{red}([H^+] < 10^(–7) M).` thus, we can summarise that Acidic solution has `color{red}(pH < 7)` Basic solution has `color{red}(pH > 7)` Neutral solution has `color{red}(pH = 7)`
Now again, consider the equation (7.28) at 298 K
`color{red}(K_w = [H_3 O^+] [OH^-] = 10^(-14))`
Taking negative logarithm on both sides of equation, we obtain
`color{red}(-log K_w = - log { [H_3O^+] [OH^-]})`
` color{red}(= - log [ H_3O^+] - log [OH^- ])`
` color{red}(= - log 10^(-14))`
`color{red}(pK_w = pH +pOH = 14)` .......(7.29)
Note that although `color{red}(K_w)` may change with temperature the variations in `color{red}(pH)` with temperature are so small that we often
ignore it.
`color{red}(pK_w) ` is a very important quantity for aqueous solutions and controls the relative concentrations of hydrogen and hydroxyl ions as their product is a constant. It should be noted that as the `color{red}(pH)` scale is logarithmic, a change in `color{red}(pH)` by just one unit also means change in `color{red}([H^+])` by a factor of 10. Similarly, when the hydrogen ion concentration, `color{red}([H^+])` changes by a factor of 100, the value of `color{red}(pH)` changes by 2 units. Now you can realise why the change in `color{red}(pH)` with temperature is often ignored.
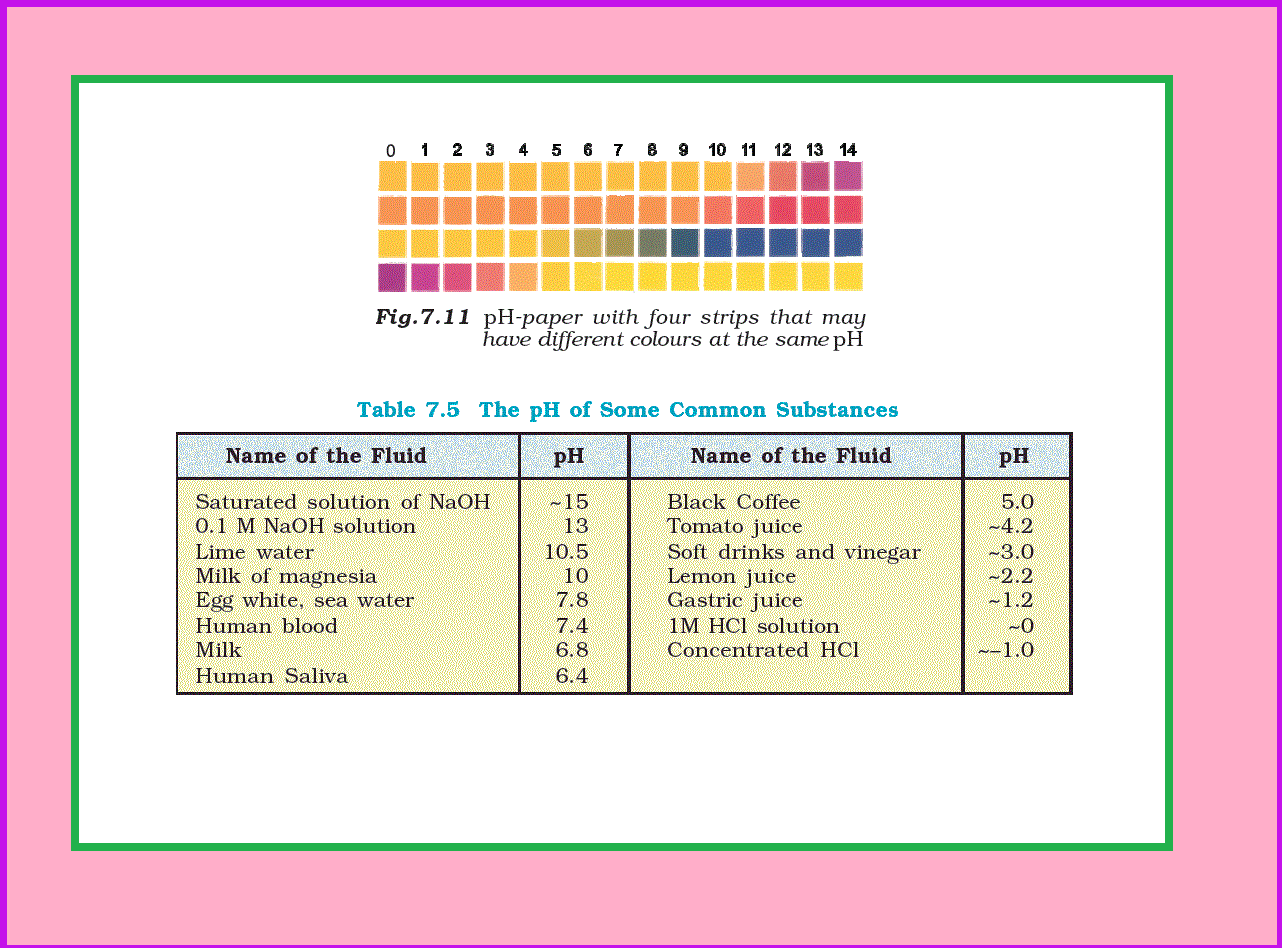
Now-a-days `color{red}(pH)` paper is available with four strips on it. The different strips have different colours (Fig. 7.11) at the same `color{red}(pH)`. The `color{red}(pH)` in the range of 1-14 can be determined with an accuracy of ~0.5 using `color{red}(pH)` paper.
For greater accuracy `color{red}(pH)` meters are used. `color{red}(pH)` meter is a device that measures the `color{red}(pH)`-dependent electrical potential of the test solution within 0.001 precision. `color{red}(pH)` meters of the size of a writing pen are now available in the market. The `color{red}(pH)` of some very common substances are given in Table 7.5 .
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
The `color{red}(pH)` of a solution is defined as the negative logarithm to base 10 of the activity `color{red}(a_(H^(+))` of hydrogen ion.
In dilute solutions `color{red}(< 0.01 M)`, activity of hydrogen ion `color{red}((H^+))` is equal in magnitude to molarity represented by `color{red}([H^+])`. It should be noted that activity has no units and is defined as:
`color{red}(a_(H^(+)) = [H^+]// mol L^(-1))`
From the definition of `color{red}(pH)`, the following can be written,
`color{red}(pH = -loga_(H^+) = - log { [H^+] // mol L^(-1) })`
Thus, an acidic solution of `color{red}(HCl (10^(–2) M))` will have a `color{red}(pH = 2).` Similarly, a basic solution of `color{red}(NaOH)` having `color{red}[OH^– ] =10^(–4) M)` and `color{red}([H_3O^+] = 10^(–10) M)` will have a `color{red}(pH = 10).`
At `25 °C`, pure water has a concentration of hydrogen ions, `color{red}([H^+] = 10^(–7) M)`. Hence, the `color{red}(pH)` of pure water is given as:
`color{red}(pH = - log (10^(-7) ) = 7)`
`=>` Acidic solutions possess a concentration of hydrogen ions, `color{red}([H^+] > 10^(–7) M)`, while basic solutions possess a concentration of hydrogen ions, `color{red}([H^+] < 10^(–7) M).` thus, we can summarise that Acidic solution has `color{red}(pH < 7)` Basic solution has `color{red}(pH > 7)` Neutral solution has `color{red}(pH = 7)`
Now again, consider the equation (7.28) at 298 K
`color{red}(K_w = [H_3 O^+] [OH^-] = 10^(-14))`
Taking negative logarithm on both sides of equation, we obtain
`color{red}(-log K_w = - log { [H_3O^+] [OH^-]})`
` color{red}(= - log [ H_3O^+] - log [OH^- ])`
` color{red}(= - log 10^(-14))`
`color{red}(pK_w = pH +pOH = 14)` .......(7.29)
Note that although `color{red}(K_w)` may change with temperature the variations in `color{red}(pH)` with temperature are so small that we often
ignore it.
`color{red}(pK_w) ` is a very important quantity for aqueous solutions and controls the relative concentrations of hydrogen and hydroxyl ions as their product is a constant. It should be noted that as the `color{red}(pH)` scale is logarithmic, a change in `color{red}(pH)` by just one unit also means change in `color{red}([H^+])` by a factor of 10. Similarly, when the hydrogen ion concentration, `color{red}([H^+])` changes by a factor of 100, the value of `color{red}(pH)` changes by 2 units. Now you can realise why the change in `color{red}(pH)` with temperature is often ignored.
Now-a-days `color{red}(pH)` paper is available with four strips on it. The different strips have different colours (Fig. 7.11) at the same `color{red}(pH)`. The `color{red}(pH)` in the range of 1-14 can be determined with an accuracy of ~0.5 using `color{red}(pH)` paper.
For greater accuracy `color{red}(pH)` meters are used. `color{red}(pH)` meter is a device that measures the `color{red}(pH)`-dependent electrical potential of the test solution within 0.001 precision. `color{red}(pH)` meters of the size of a writing pen are now available in the market. The `color{red}(pH)` of some very common substances are given in Table 7.5 .



